Extraction and Socket Grafting: Part 2 — Extraction Site Healing
In Part 1 of this three-part series on extraction and socket grafting, the importance of atraumatically removing a tooth while maintaining the surrounding hard and soft tissue was explained. In this article, Part 2 of the series, we will discuss extraction site healing, along with indications and contraindications for socket grafting. Part 3 will be a comprehensive description of the step-by-step socket grafting protocol, which will be discussed with respect to the type of bone grafting material indicated and the use of various membranes in the bone regeneration process.
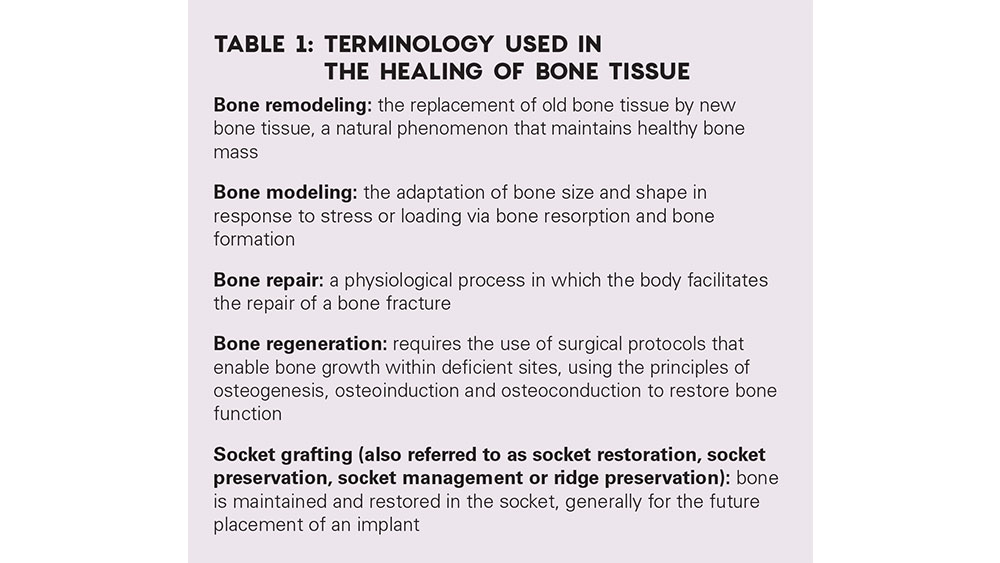
To understand how healing occurs at an extraction site, the clinician requires a comprehensive understanding of the terminology used when describing two distinct processes that occur after a tooth is removed — repair and regeneration (Table 1). Bone repair is a physiological process in which the body repairs bone fractures. This most often occurs when the labial plate of bone is lost as a consequence of the extraction. In contrast, bone regeneration occurs most commonly when all bony walls are present after extraction, as healing occurs by secondary intention. The healing sequence of hard and soft tissue after extraction includes several processes, including inflammation, epithelialization, fibroplasia and remodeling.
Our profession has seen significant advancements in the understanding of bone healing at the cellular and molecular level, which has led to techniques that have expanded the scope of oral implantology. In most cases, the extraction process will initiate a sequence of bony resorptive morphologic changes that may negatively alter the alveolar ridge. These changes are variable and, if significant, can eliminate the possibility of future implant placement. Therefore, it is imperative clinicians understand these concepts and apply them to diagnosis and treatment planning with respect to the treatment of extraction sites.
TO GRAFT OR NOT?
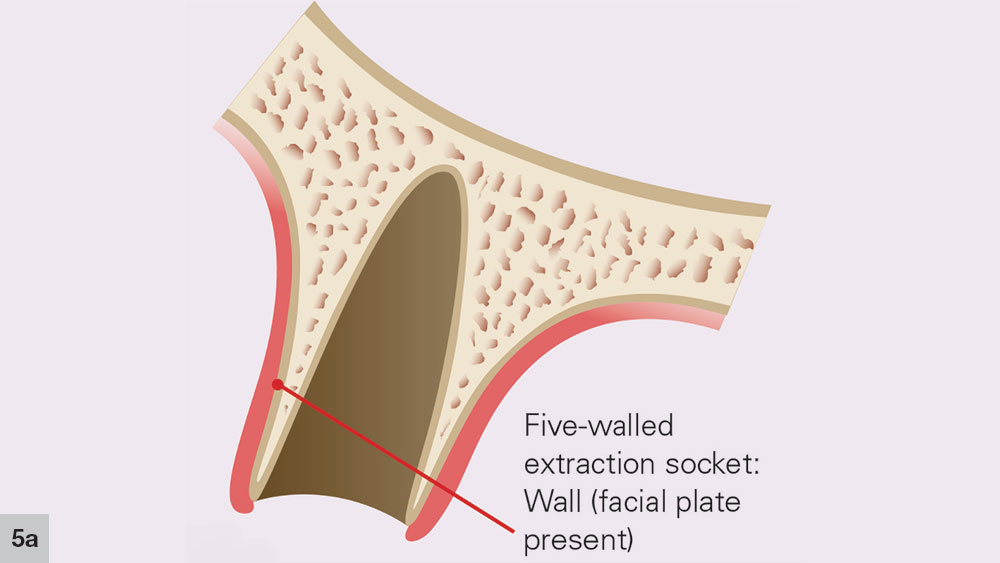
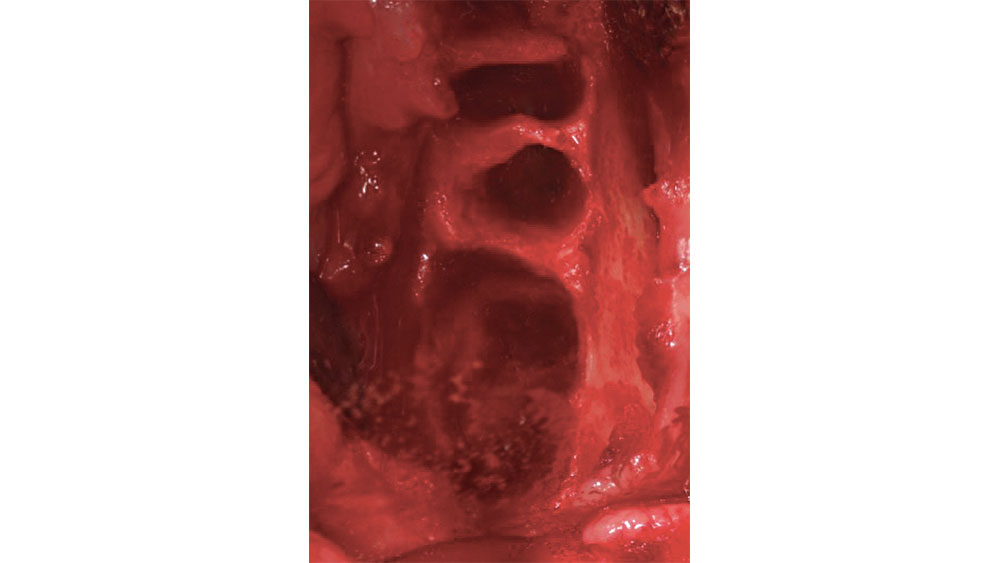
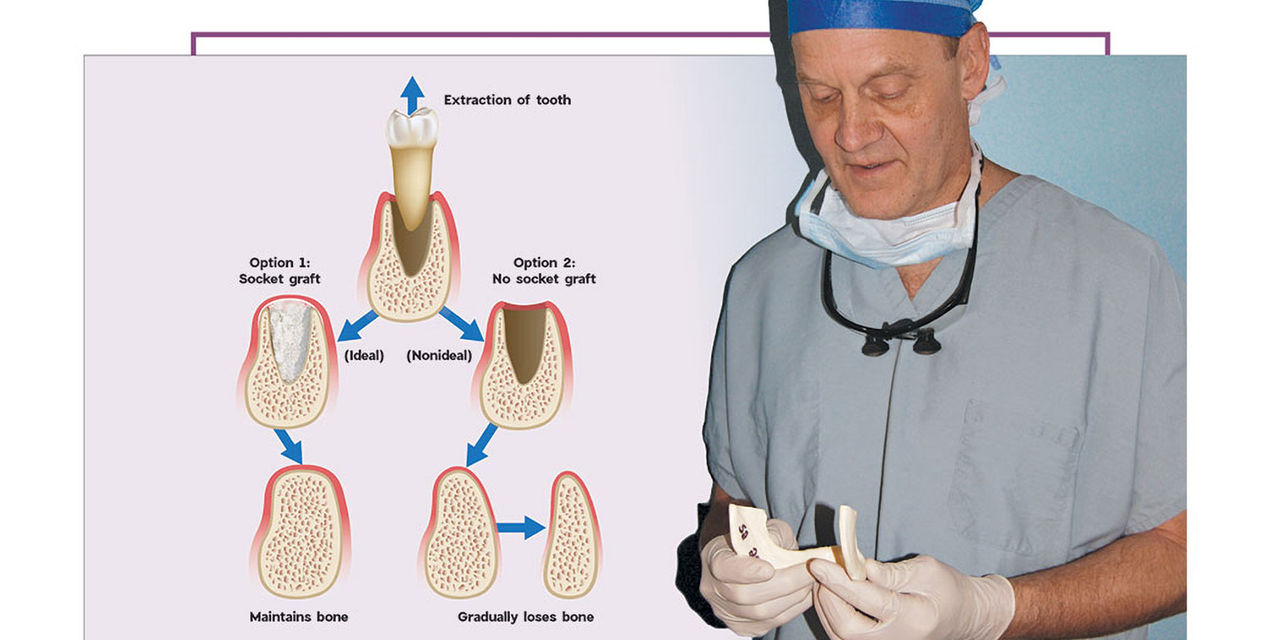
After a tooth extraction, the clinician is presented with various options including immediate implant placement, socket grafting, or no socket grafting. If all socket walls are present and the surgical anatomy is ideal, immediate replacement is often the preferred method of treatment. However, if an implant will not be placed immediately, the clinician should evaluate and classify the extraction site based on the number of walls remaining. A tooth socket contains five walls: the mesial, distal, buccal, lingual and apical.1 The number of walls remaining dictates the treatment choice: to graft or not to graft (Fig. 1).
Figure 1: The determination of whether or not to graft is based on an evaluation and classification of the extraction site; the number of tooth socket walls remaining will dictate the treatment. When grafting is recommended, the Newport Biologics™ line of regenerative materials (Glidewell Direct; Irvine, Calif.) helps dentists simplify the procedure.
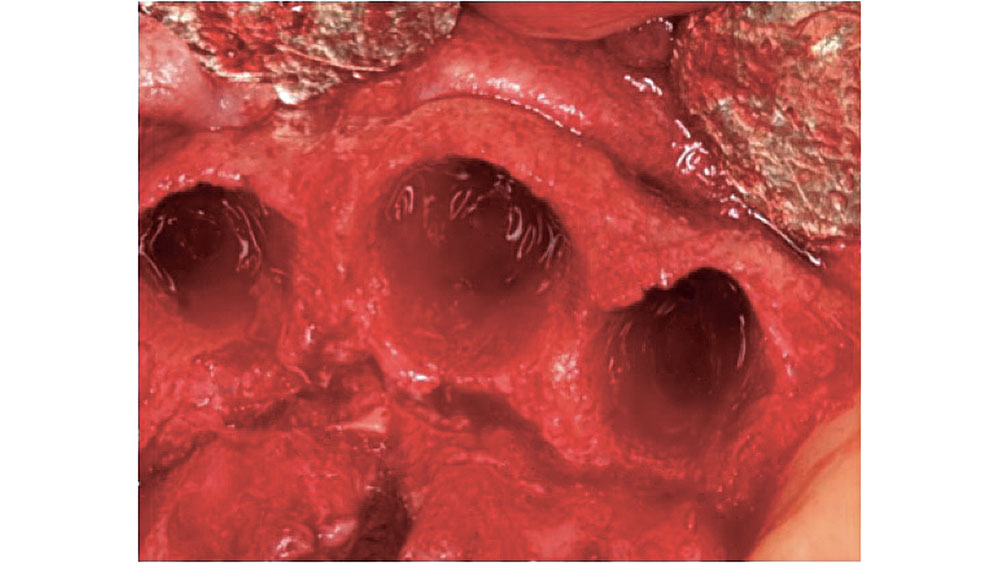
Figure 2: Five-walled extraction socket (mesial, distal, buccal, lingual, apical).
FIVE-WALLED EXTRACTION SOCKET (THICK BONY WALLS)
In a five-walled socket, all the bony walls are present. Therefore, this type of extraction socket is the most predictable to treat. If the walls are thick (greater than 1.5 mm) and have the interseptal bone remaining, then grafting of the socket is not necessarily required (Fig. 2).
In most five-walled sockets, healing is completed via bone regeneration, mainly by secondary intention. This type of healing is similar to secondary intention soft-tissue healing. If the tooth extraction was atraumatic, most of the keys for bone regeneration are present for a predictable result. The five-walled extraction site sets up a regional acceleratory phenomenon (RAP) for healing, which increases the regeneration process and adds growth factors such as bone morphogenetic protein (BMP) to the site.
When grafting is indicated, which is typically the case when the buccal plate is less than 1.5 mm thick, the five bony walls help protect the graft from mobility, allowing for undisturbed healing. The torn blood vessels in the periodontal complex leak growth factors, including platelet-derived growth factor (PDGF) and transforming growth factor (TGF), into the region, further accelerating the healing process. The five bony walls also allow for space maintenance, which is a key component of predictable hard- and soft-tissue healing. The other key to bone regeneration is soft-tissue healing. The soft tissue surrounding an extraction site begins to heal over the clot and granulation tissue of the socket within two or three weeks.
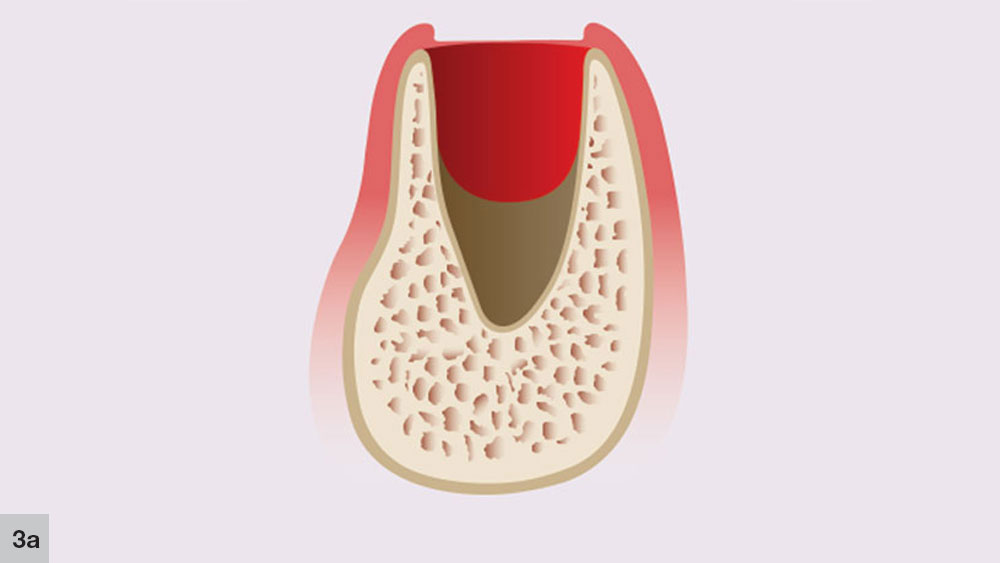
In a five-walled socket, healing occurs in a five-stage process. The concept of the five stages of bone healing was initially proposed by Ohta in animal studies, and was then extrapolated to humans.2 The first stage, the granulation stage occurs immediately at the time of tooth extraction and lasts up to five days (Fig. 3a). This stage includes the formation of granulation tissue, which appears at the socket apex and spreads laterally and crestally along the socket walls.
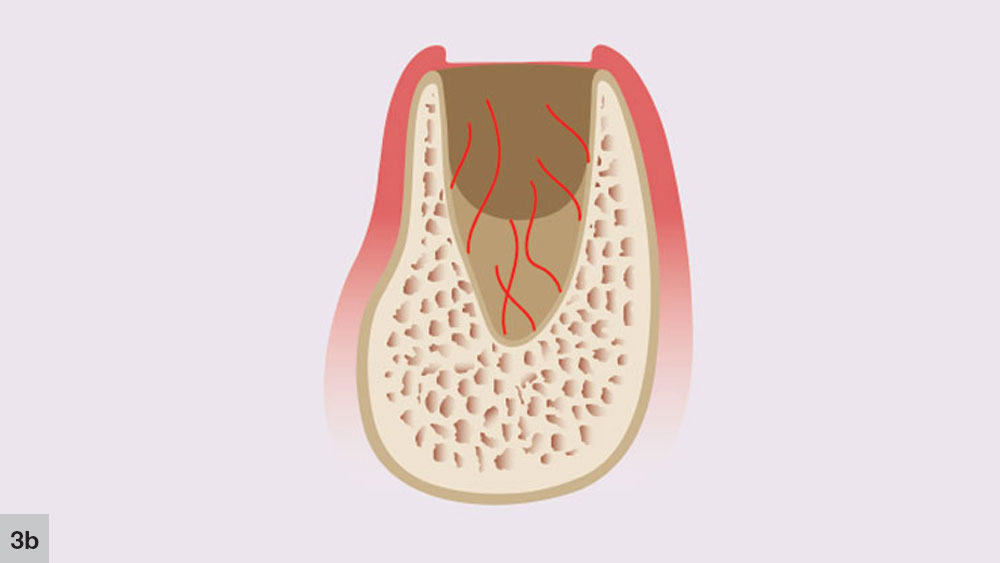
The second phase takes place concurrently with the first stage. The initial angiogenic stage occurs with the formation of new blood vessels that develop from the broken ends of blood vessels in the residual periodontal ligament that covers the cribriform plate (Fig. 3b). Blood plasma will leak from the severed blood vessels, which allows immature fibroblasts to aggregate at the plasma-rich regions. New trabeculae then may develop at the socket apex and the center of the blood clot, which begins to shrink.
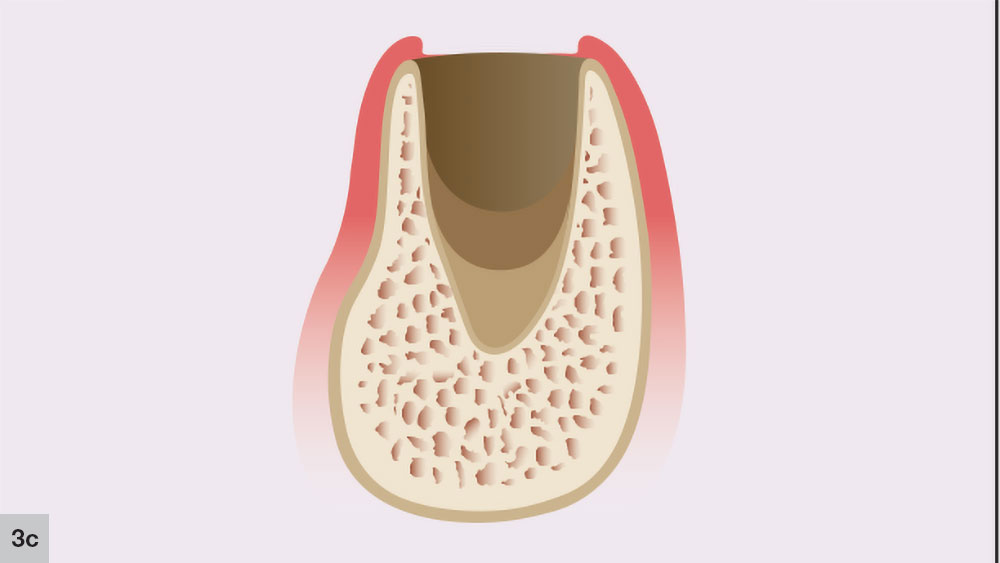
The third phase of socket healing is the new bone formation stage, which starts approximately three to four weeks after extraction (Fig. 3c). The extraction site is filled with granulation tissue and the capillary activity begins the early phases of bone trabeculae development. This capillary activity is initiated at the socket apex, and trabeculae of woven bone growth will occur following the formation of blood vessels. During this stage, the cortical bone of the crestal area of the socket will resorb, along with the interseptal regions and the thinner facial plate.
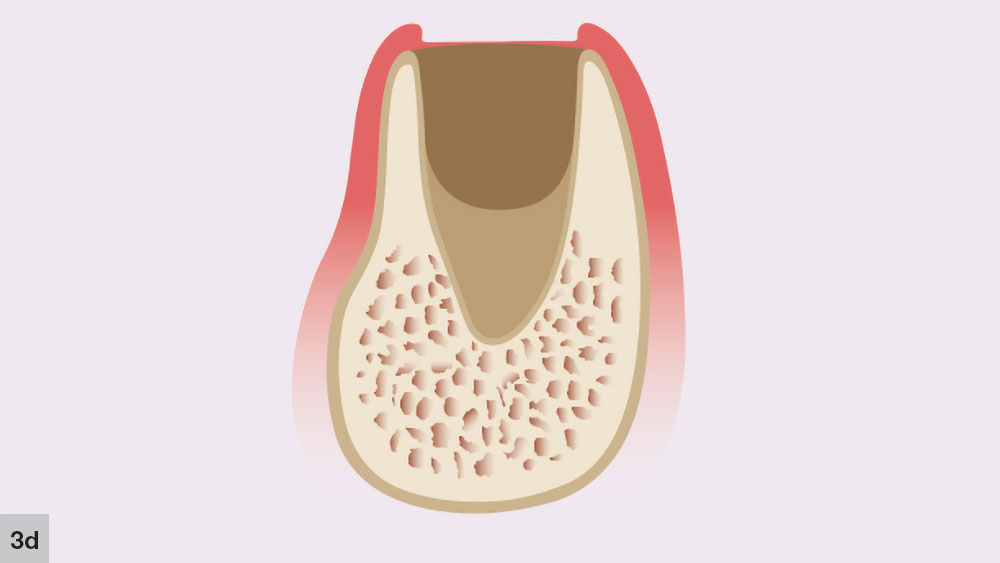
The fourth phase, the bone growth stage, starts around four to six weeks after the extraction (Fig. 3d). New bone trabeculae form on the walls and approximately two-thirds of the socket. At this stage, the center of the socket is primarily composed of woven bone. The more-organized lamellar bone starts to form from the lining of the socket, moving toward the center.
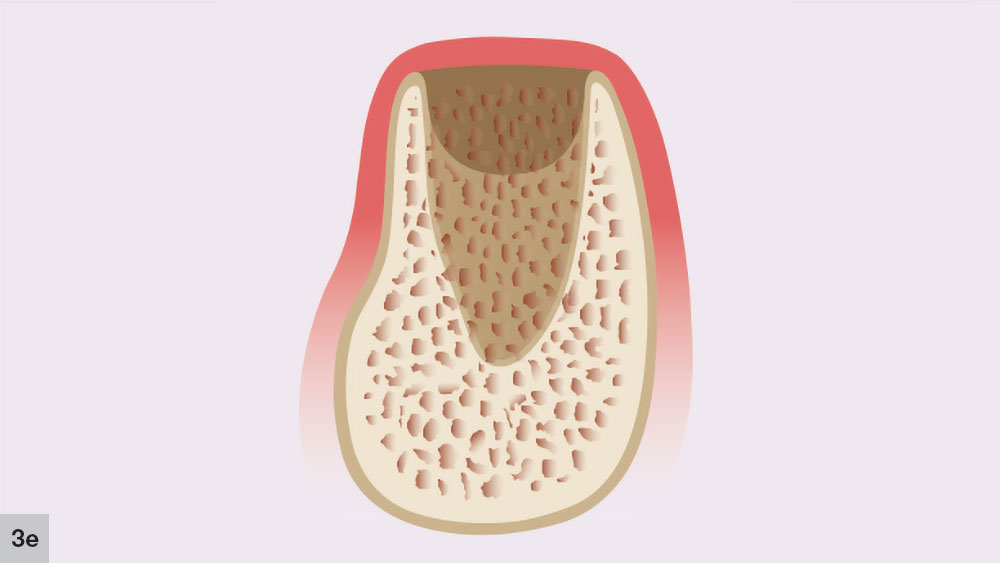
The bone reorganization stage, which is the final phase, occurs approximately six weeks after extraction and may last up to four months (Fig. 3e). In this stage, the primary bone trabeculae remodel to form thicker secondary cancellous bone. This remodeling process will continue for approximately four to six months after the initial extraction.
The timing for these five stages varies among individuals and clinical situations. The number of bony walls around the socket and size of the alveolus greatly influence the regeneration process. In general, larger sockets, such as molar sites, take longer to completely form bone compared with smaller-diameter anterior sites.2
FIVE-WALLED EXTRACTION SOCKET (THIN BONY WALLS)
With some five-walled extraction sockets, one or more of the bony walls may be considered thin (less than 1.5 mm). This is most common in the maxillary anterior region, as the average thickness has been shown to be less than 1 mm.3 Therefore, in these types of sites, grafting of the socket is recommended regardless of the anatomic location. Because of the bony wall thinness, the predictability of maintaining the socket volume is reduced. The space is less likely to be maintained and the vascularization is reduced, leading to delayed healing and increased bone loss.
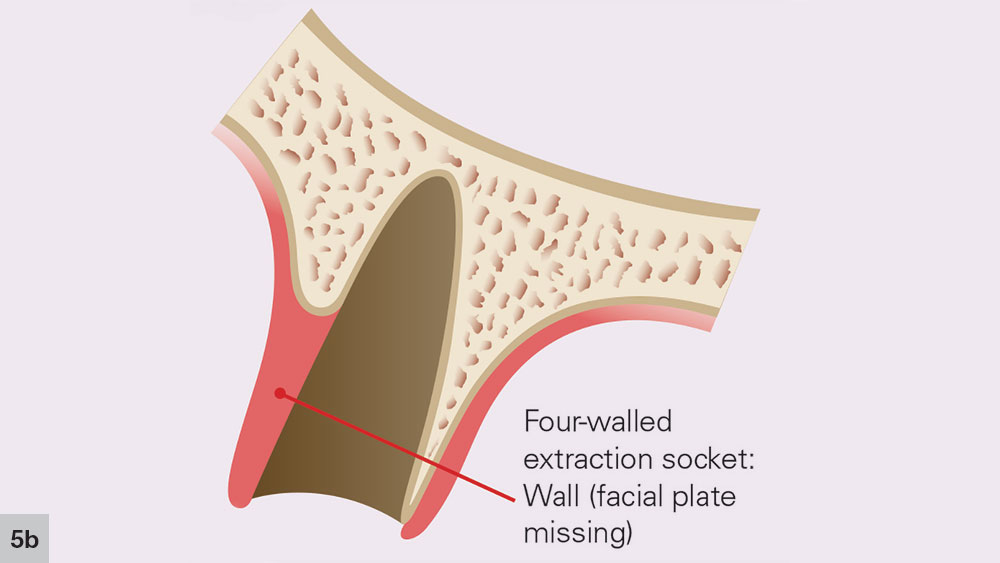
FOUR-WALLED EXTRACTION SOCKET
With a four-walled extraction socket, one wall is missing — typically the buccal or facial plate (Fig. 4). Unlike a five-walled socket, which heals through bone regeneration, a four-walled socket will most likely heal via bone repair, compromising the predictability of the bone healing process (Figs. 5a, 5b). When the buccal wall is missing, many required elements of the bone regeneration process are lost. The loss of the buccal wall will reduce the ability of the space to be maintained. Because space maintenance is required for predictable bone growth, an increased amount of bone loss most likely will occur, along with a decrease in host-bone vascularization. This may lead to reduced blood supply for bone formation and bone growth factors entering the site.
The fourth phase, the bone growth stage, starts around four to six weeks after the extraction (Fig. 3d). New bone trabeculae form on the walls and approximately two-thirds of the socket. At this stage, the center of the socket is primarily composed of woven bone. The more-organized lamellar bone starts to form from the lining of the socket, moving toward the center.
The bone reorganization stage, which is the final phase, occurs approximately six weeks after extraction and may last up to four months (Fig. 3e). In this stage, the primary bone trabeculae remodel to form thicker secondary cancellous bone. This remodeling process will continue for approximately four to six months after the initial extraction.
The timing for these five stages varies among individuals and clinical situations. The number of bony walls around the socket and size of the alveolus greatly influence the regeneration process. In general, larger sockets, such as molar sites, take longer to completely form bone compared with smaller-diameter anterior sites.2
Source: Sleep Review: The journal for sleep specialists [internet]. Leawood (KS): Anthem Systems; c2019. Nearly 1 Billion People Worldwide Have Sleep Apnea, International Sleep Experts Estimate. 2018 May 22 [cited 2019 Feb 4]. Available from: https://www.sleepreviewmag.com/2018/05/nearly-1-billion-people-worldwide-sleep-apnea-international-sleep-experts-estimate/.
In four-walled extraction sockets, grafting is indicated to reduce residual bone and soft-tissue loss. The maxillary anterior region may resorb up to 23 percent in the first six months after an extraction, and an additional 11 percent over the following five years.4 Within 24 months, an average of 40 to 60 percent of the preexisting height and width of bone may be lost with this type of extraction socket.5
THREE-, TWO- AND ONE-WALLED EXTRACTION SOCKETS
When more than one bony wall is missing, there is a greater need for extensive bone grafting, which has traditionally involved the use of autogenous bone harvested from the mandibular ramus, mandibular symphysis or maxillary tuberosity. Increasingly, these defects are grafted with allograft to simplify the surgical procedure. If bone grafting in these types of extraction sockets is not performed, extensive loss of bone will result, along with soft-tissue loss, which leads to esthetic issues. Three-, two- and one-walled defects are highly compromised situations that usually involve through-and-through defects and are highly unpredictable (Fig. 6).
GRAFTING INDICATION SUMMARY
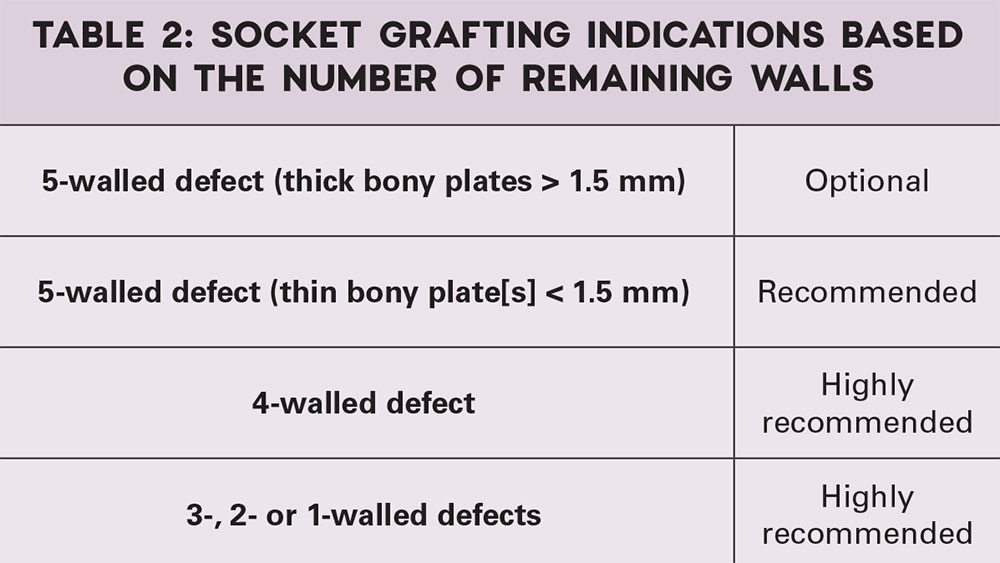
When all five walls of the socket are present, but one or more walls are thin (less than 1.5 mm), socket grafting is indicated. When additional walls are missing, socket grafting is highly recommended (Fig. 7).
CONTRAINDICATIONS TO SOCKET GRAFTING
Bone grafting is contraindicated in some situations, such as in the case of active infection or an otherwise compromised site.
Infected Site
A relative contraindication to the socket grafting protocol is the presence of an acute infection that cannot be remediated. A tooth demonstrating active infection (i.e., exudate or fistula), which may show residual infection, should be extracted without placement of bone graft material. An acute infection that cannot be completely removed should be managed by drainage of the infection, lavage of the infected area, and the use of systemic antibiotics. The bone grafting may be delayed for approximately eight to 12 weeks post-extraction to decrease the possibility of an acute infection. This delay will also ensure better tissue quality as a result of primary closure of the grafted site with keratinized tissue, as well as elevated osteoblast activity and a newly formed woven bone interface within the socket. The disadvantage of this surgical approach is the need for an additional surgery, which increases the treatment time and patient discomfort.
Mandibular Premolar Compromised Sites
In the mandibular premolar area, 25–38 percent of the time, the mental foramen is superior to the apex of either of these teeth.6 The location of the mental foramen is highly variable, but most commonly located in the 1st or 2nd premolar area. If a premolar is indicated for extraction, CBCT measurements should be completed to confirm adequate bone below the apex. The clinician must be careful in debriding this area as nerve impairment may occur (Fig. 8).
In the mandibular molar area, especially in Type 1 nerve paths, where the mandibular canal is near the mandibular teeth apexes, it is important not to curette or place graft material in close proximity to the nerve canal. Trauma from curettage or direct placement of graft material into the nerve canal may cause a temporary or permanent neurosensory impairment.
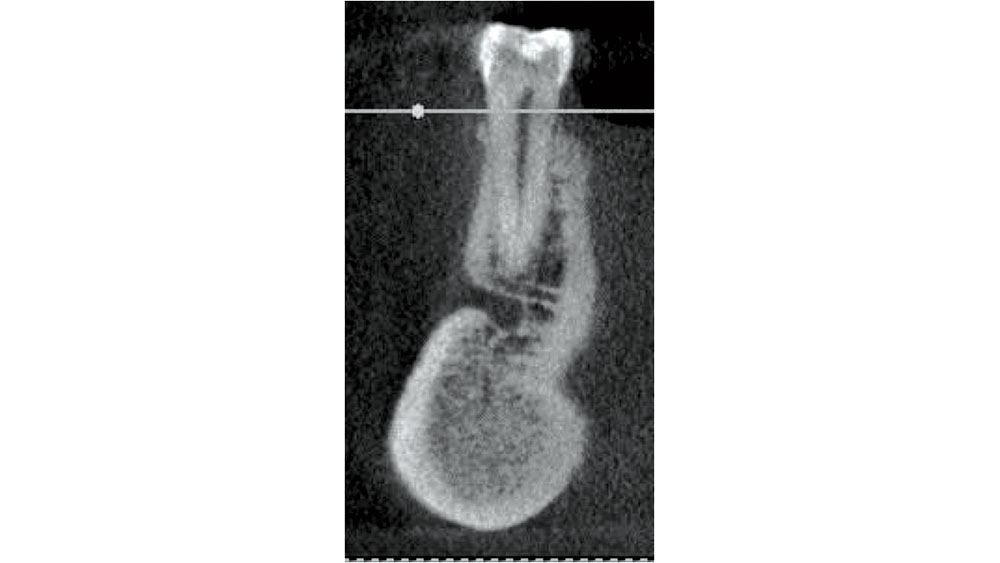
Figure 8: Mandibular premolar proximity to mental foramen.
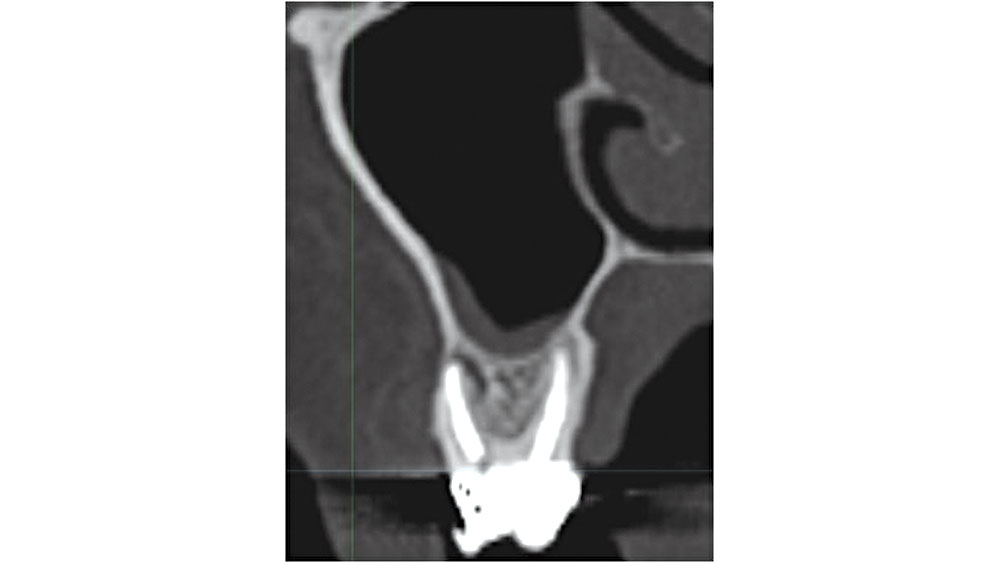
Figure 9: Maxillary molar proximity to maxillary sinus.
Maxillary Compromised Sites
In the maxillary molar areas, approximately 42 percent of the roots extend into the sinus proper.7 When the tooth is extracted, the sinus membrane may be perforated and an exposure may occur. If grafting is completed, bone graft material may be introduced into the sinus, leading to the possibility of an acute rhinosinusitis. In addition, care should be exercised in curetting soft-tissue lesions, as complications may result (Fig. 9).
CONCLUSION
A classification of the indications for socket grafting has been discussed with respect to the remaining socket walls. When all walls of the socket are present, and the bony walls are thick, grafting of the extraction socket is optional. However, when all the walls of the socket are present, but one or more walls are thin (less than 1.5 mm), socket grafting is indicated. When additional walls are missing, socket grafting is highly recommended (Table 2). In general, the more walls compromised or missing, the greater the need for grafting. In these types of situations, the use of bone growth factors, such as bone morphogenic protein, is indicated.
Earn free CE credit for this article. Go to glidewelldental.com/1402-ce to enter your answers.
Available CE Course
References
- ^ Misch CE, Suzuki JB. Tooth extraction, socket grafting, and barrier membrane bone regeneration. In: Misch CE, editor. Contemporary Implant Dentistry, 3rd ed. St. Louis: Mosby; 2008. p. 870-904.
- ^ Ohta Y. Comparative changes in microvasculature and bone during healing of implant and extraction sites. J Oral Implantol. 1993;19(3):184-98.
- ^ Vera C, De Kok IJ, Reinhold D, Limpiphipatanakorn P, Yap AK, Tyndall D, Cooper LF. Evaluation of buccal alveolar bone dimension of maxillary anterior and premolar teeth: a cone beam computed tomography investigation. Int J Oral Maxillofac Implants. 2012 Nov-Dec;27(6):1514-9.
- ^ Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: histomorphometric evaluations at 9 months. J Periodontol. 2000 Jun;71(6):1015-23.
- ^ Wang HL, Kiyonobu K, Neiva RF. Socket augmentation: rationale and technique. Implant Dent. 2004 Dec;13(4):286-96.
- ^ Fishel D, Buchner A, Hershkowith A, Kaffe I. Roentgenologic study of the mental foramen. Oral Surg Oral Med Oral Pathol. 1976 May;41(5):682-6.
- ^ Kang SH, Kim BS, Kim Y. Proximity of posterior teeth to the maxillary sinus and buccal bone thickness: a biometric assessment using cone-beam computed tomography. J Endod. 2015 Nov;41(11):1839-46.